Automated dressing
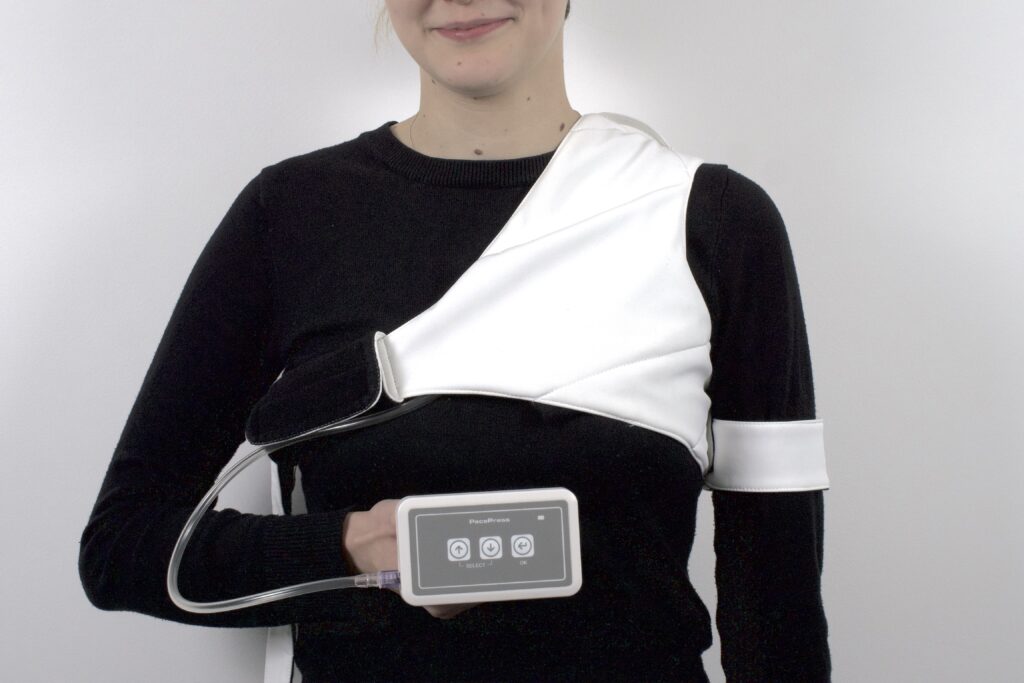
PacePress is a pneumatic compression dressing designed to minimize the risk of bleeding complications in patients after CIED (Cardiovascular Implantable Electronic Device) implantation procedures, while ensuring patient comfort.
Risk reduction
Approximately 2-10% of CIED* device implantation procedures are accompanied by a complication in the form of a hematoma after implantation of a medical device used in cardiac electrotherapy, which may lead to infection and the need for reoperation.
* Cardiac Implantable Electronic Devices – implantable cardiovascular electronic devices, e.g.: pacemaker, cardioverter-defibrillator, or cardiac resynchronization therapy device.
Why is it worth it?

reducing the risk of complications

improving the comfort of patient mobility

lower patient treatment costs

easy to put on
and adjust the cuff

monitoring device parameters

Patent protection
PacePress consists of a pumping unit, a pneumatic cushion and a compression cuff with a harness. Adjustable straps allows to adjust the cuff to the individual needs and anatomy of the patient. The device has automatic pressure regulation, controlled by an easy-to-use interface. The pumping unit is located in a light and handy housing. Thanks to the clip, it is possible to conveniently attach the device.
PacePress market
It is estimated that over 1.5 million heart electrotherapy device implantation procedures are performed worldwide each year. With the increasing number of more complicated CIED implantation procedures and reoperations, which increasingly concern older people with numerous comorbidities, the number of complications of these procedures is increasing. According to various studies, clinically significant hematomas of the bed occur in 2-10% of all procedures. It is estimated that almost 35% of patients undergoing CIED implantation or replacement require long-term use of agents affecting body homeostasis, antiplatelet drugs and/or anticoagulants. Moreover, this percentage continues to increase with the aging of societies, which implies the spread of indications for chronic antiplatelet and anticoagulation therapy.
Project progress
The project is undergoing clinical trials, during which improved versions of compression cuffs are also being tested. Currently, research is being conducted in six centers throughout Poland. Additionally, in order to increase the dynamics of patient recruitment, changes were introduced to the study protocol. Based on feedback from users, i.e. doctors and patients, the Company’s engineering team is developing a newer version of the control unit with the ability to aggregate and transmit data via a mobile application. After completing the clinical trials and receiving the official report with the results, Medinice plans to start the CE certification process according to the new MDR standards.
* The aforementioned project signifies a product that has not yet received approval as a medical device from the relevant regulatory authority. Competitive advantages and other aspects of the aforementioned project will be substantiated thorough testing and research.






