
PacePressSmart pressure
Confident recovery
About the product
PacePress is a medical device designed to reduce the risk of complications following the implantation of cardiac electrotherapeutic devices, such as pacemakers or implantable cardioverter-defibrillators (ICDs). It helps prevent postoperative internal bleeding, a serious complication to patients that also increases treatment costs. The device is simple to use, does not need any adjustments, and once explained in the hospital, patients can easily continue using it on their own at home.
The Problem
The Problem
Approximately 1.5 million Cardiac Implantable Electronic Device (CIED) procedures are performed globally each year, including 50,000 in Poland
About 60% of patients undergoing CIED implantation are at risk of developing postoperative complications such as:
- hematoma
- infection
- need for reoperation
Current postoperative care standards do not eliminate the risk of complications
These standards typically include:
- immobilization of the patient in a lying position for at least one day
- applying pressure to the implantation site using sandbags or weighting it with cold compresses
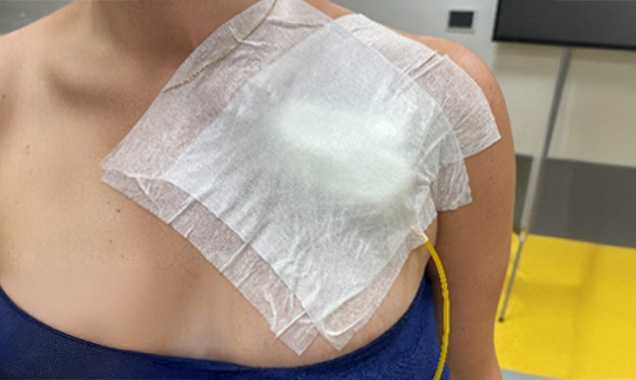
Current prevention does not guarantee the absence of complications
FOR THE PATIENT
- standard prophylaxis is associated with patient discomfort, including immobilization and pain during even the simplest activities
- postoperative complications may be associated with discomfort and additional interventions
- the management of complications prolongs the patient's hospital stay
FOR THE HOSPITAL
- the patient requires continuous nursing supervision following surgery
- postoperative complications such as hematomas typically occur during the patient's hospital stay
- the treatment of complications after CIED implantation increases hospital costs due to the prolonged length of hospitalization
The Solution
The Solution
PacePress provides faster recovery after CIED implantations
PacePress is a medical device designed to minimize the risk of complications and reoperations, providing comfort to the patient.
A medical device designed to minimize the risk of complications and reoperations, providing comfort to the patient, through automatic compression of the implantation site of CIED.
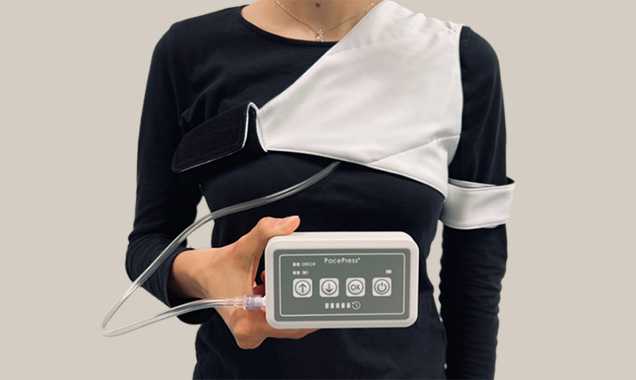
Patented design and functionalities
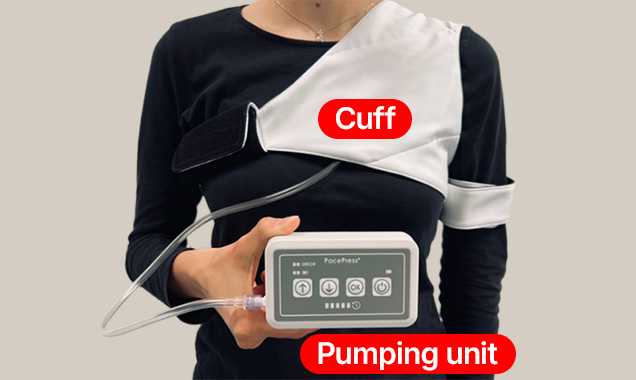
Key elements of PacePress are the cuff and the pumping unit.
The ergonomic harness allows for the adjustment of the cuff to the individual needs and anatomy of the patient.
The pumping unit is housed in a lightweight and portable casing. The device features pressure regulation, controlled via an easy-to-use touchscreen panel.
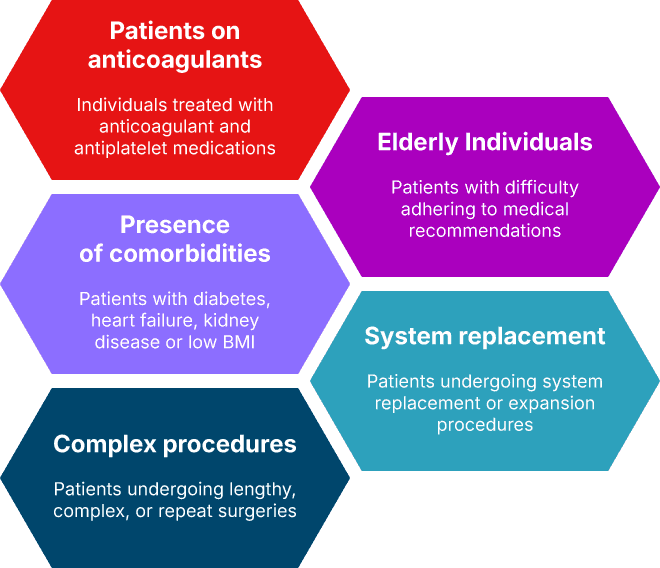
Risk groups
PacePress user risk groups
PacePress is recommended for several risk groups, which together make up approximately 60% of all patients. These include individuals:
- taking anticoagulant or antiplatelet medications (49%)
- older adults – simple design helps ensure proper use after brief guidance
- with comorbidities such as diabetes, heart failure, kidney failure, or a BMI below 23 kg/m²
- undergoing revision or reconstruction surgery (29%)
- facing a long or complex procedure, or undergoing reoperation within three months
Global Patent Protection
Global Patent Protection
Our PacePress technology is protected by a wide patent portfolio, highlighting our commitment to innovation. Patents granted in key areas such as Poland, Canada, China, Japan, South Korea, EPO, and India. PacePress is protected by a comprehensive and wide-ranging intellectual property protection strategy. This broad protection ensures uniqueness, reliability, and competitive advantage of our technology worldwide.

PacePress key features
PacePress key features
- Reduces the risk of hematoma and infection in the postoperative cavity
- Supports adherence to local compression guidelines in the early period after CIED implantation
- LED indicators facilitate the control of compression time after the procedure

PacePress key features - continued
- Potential reduction in hospital stay
- Allows for continued compression for up to four days in home conditions
- Increases control, safety, comfort, and mobility of the patient
We Care About Your Privacy
Medinice S.A. uses cookies to improve and customize users experience on our website. By selecting 'Accept', you consent to the use of all cookies that gather and use information about your interactions with our site to provide personalized content and enhance your digital experience. Please read our Privacy Policy for more information.