Medinice receives positive FDA feedback in the 510(k) process for CoolCryo
August 26, 2025
Medinice S.A. announced another important milestone in the U.S. registration of its CoolCryo system. The company received official comments from the FDA regarding its 510(k) submission. The Agency’s remarks are limited to documentation clarifications, with no requirement for repeated studies or additional external testing.
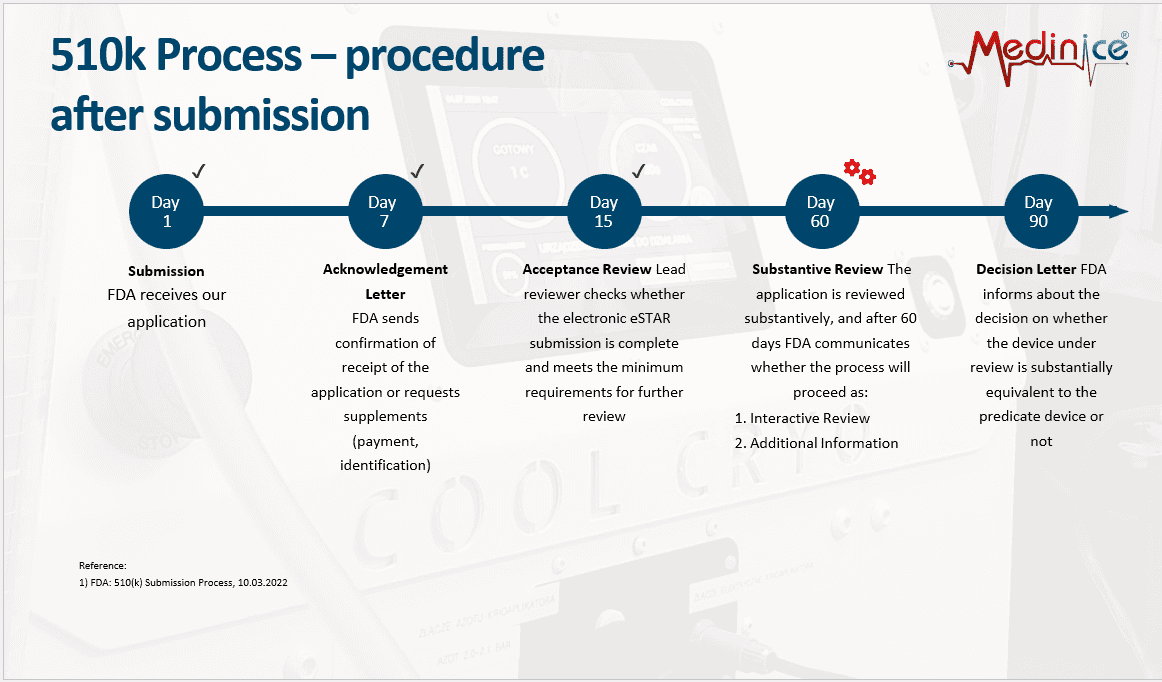
This is highly positive news, confirming that the project is progressing through the substantive review stage on schedule and moving closer to final approval and US market entry.
“This is an important step forward that brings us closer to the approval of CoolCryo for sale in the US” - said Sanjeev Choudhary, CEO of Medinice.
Detailed information on the CoolCryo technology can be found under the following link:
Learn moreWe Care About Your Privacy
Medinice S.A. uses cookies to improve and customize users experience on our website. By selecting 'Accept', you consent to the use of all cookies that gather and use information about your interactions with our site to provide personalized content and enhance your digital experience. Please read our Privacy Policy for more information.